Cell and gene therapy will be top industry trend for pharma in 2024
BioPharma Reporter
JANUARY 8, 2024
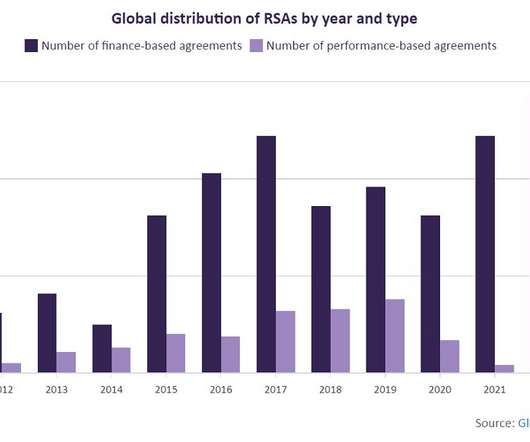
Healthcare industry professionals scored cell and gene therapy (CGT) as the industry trend to have the greatest impact on the pharmaceutical industry in 2024, in a recent survey launched by data and analytics firm GlobalData.





























Let's personalize your content